Every software vendor, healthcare institution and pharmacy that displays medicine information will be legally required to switch to the digital ePI standard (XML/FHIR).
Important information on behalf of Leaphy, a digital health technology company specialised in enabling technical compliance with the EU electronic Product Information (ePI) Common Standard and IDMP/SPOR frameworks.
As the EU moves toward mandatory ePI implementation (2025–2027), many Regulatory Information Management (RIM) and IDMP platforms are now looking for reliable, standards-aligned components that can transform approved product information into machine-readable, accessible, and resolvable ePI.
Leaphy’s platform is designed precisely for this:
• PDF → XML/FHIR conversion aligned to EMA/HMA Common ePI Standard and SPOR/IDMP master data.
• Automated validation engine ensuring conformance with the ePI schema and metadata rules.
• Repository and version management with audit trail and approval workflows.
• Resolver service connecting GTINs, NTINs, and national codes from the existing GS1 DataMatrix 2D code on packs to the correct ePI endpoint — no packaging changes required.
• API and integration layer for embedding directly within RIM or submission systems.
We see strong potential for collaboration — either as an OEM integration, API partnership, or co-marketed compliance module that enhances your RIM suite’s ePI readiness.
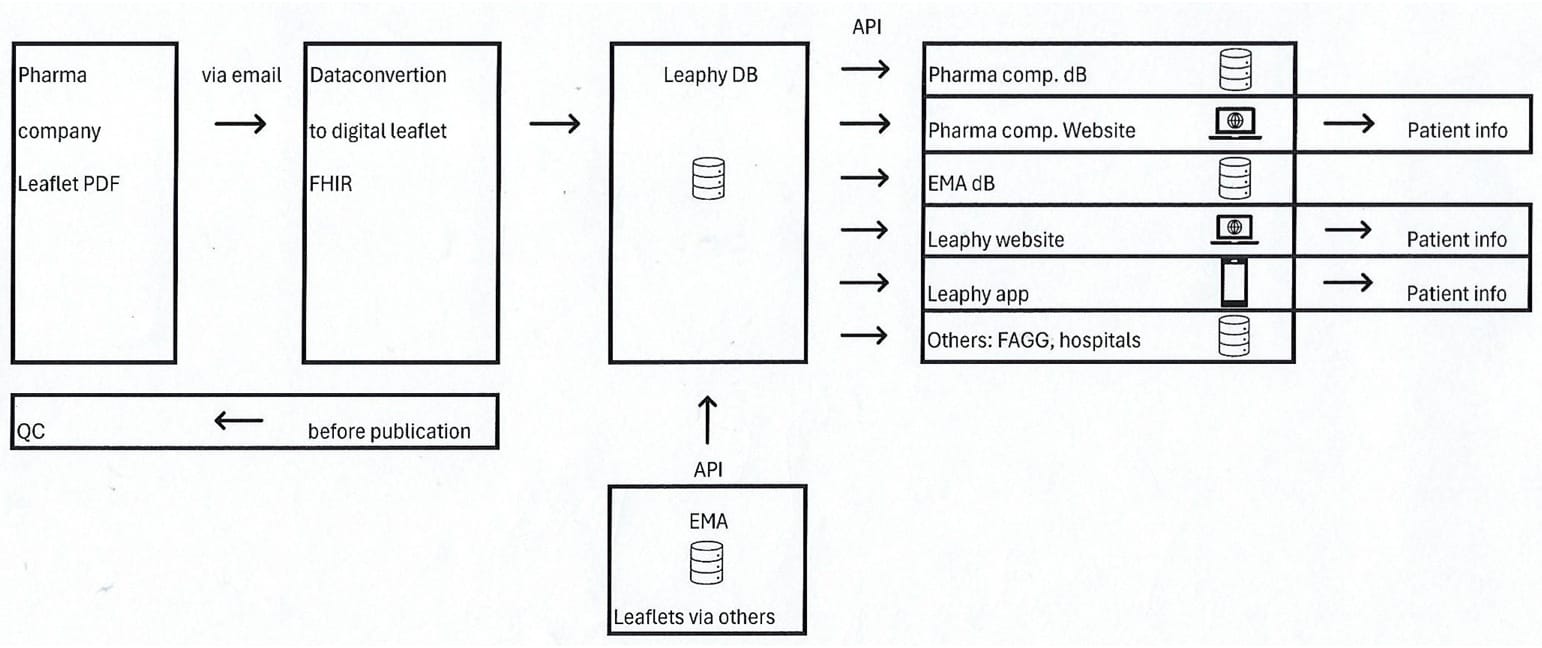
• See also dataflow below
Please do not hesitate to contact us with the contact form for any help on digitalisation of your leaflets and importing them into the EMA database.
Leaphy® is your gateway to effective leaflet management and digital transformation, specifically tailored for e-Pil.
Our comprehensive leaflet management platform empowers you with precise e-Pil administration across all EU countries and ensures versatile access to e-Pil through multiple channels.
Our diverse Leaphy® functionalities will be available in all European languages, reflecting our commitment to making European leaflets accessible to both patients and healthcare professionals. As a pharmaceutical company, you can leverage enabled push technology to keep patients and physicians informed about medication updates.
Leaphy® diligently transforms pharmaceutical leaflets into a structured data format, optimizing their usability. Data is securely stored within an Azure database environment, offering the flexibility of daily updates to the pharmaceutical company database. We also prioritize aligning metadata standards with those required to comply with the Falsified Medicines Directive (FMD) and ensure adherence to ISO IDMP standards.
You can access all leaflet data via the Leaphy app or an API
This data can also be transmitted through an API to the company database environment or website.
Leaphy® extends a user-friendly interface to patients and healthcare providers, offering easily digestible public leaflet information (PIL). Furthermore, pharmaceutical companies can supplement leaflets with additional digital content such as videos or audio, enhancing the overall patient experience.